Did you know that over 70% of biopharmaceutical products are sensitive to temperature fluctuations during transportation? This staggering fact highlights the critical need for robust systems like the antibody Registration system, which ensures that these vital components remain stable and effective throughout their journey!
The Marvels of Antibody Registration Systems
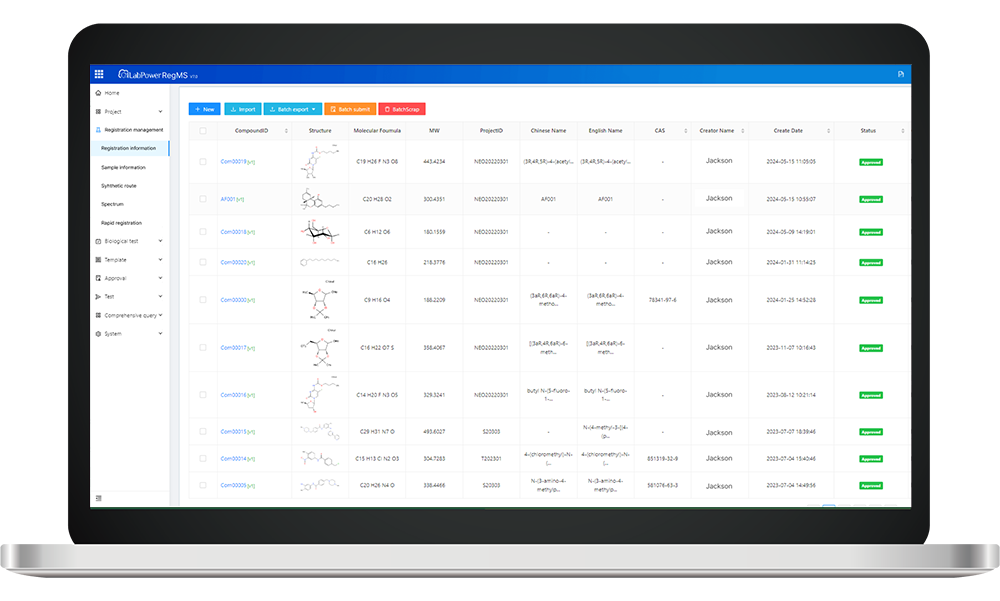
The antibody registration system is a game-changer in managing and tracking antibodies from production to delivery. Its unique transport attributes ensure that each shipment adheres to stringent environmental conditions, safeguarding the integrity of these life-saving molecules. One standout feature is its ability to facilitate Carrier Compliance Audits, ensuring all carriers meet regulatory standards while transporting sensitive biological materials. With this system in place, we can confidently navigate the complexities of logistics without compromising quality.
Diving Deeper into R&D Project Management Software and Carrier Compliance Audits
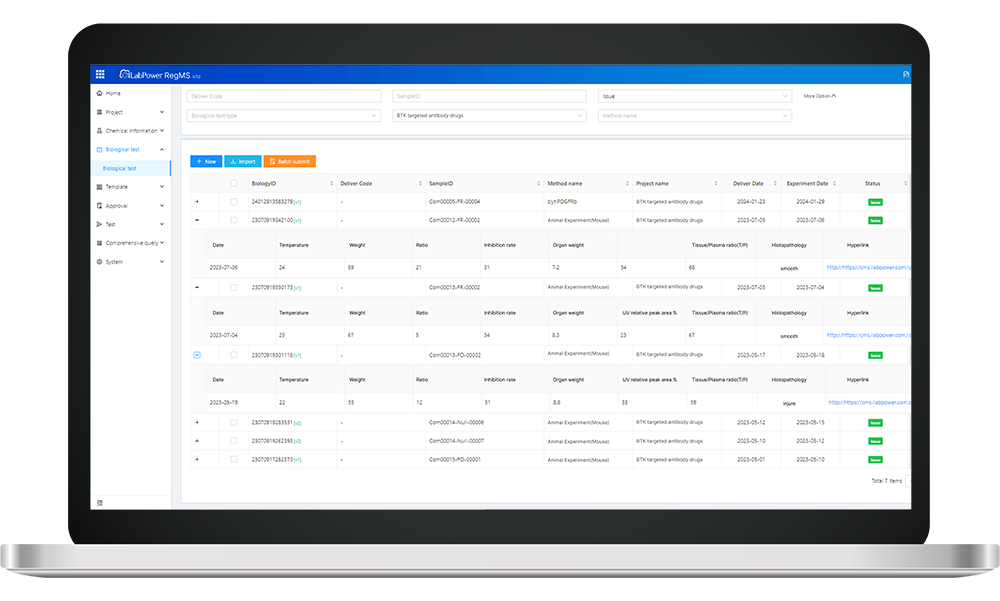
When it comes to R&D project management software integrated with carrier compliance audits, we’re talking about an innovative approach that streamlines processes while enhancing accountability! This software allows researchers and logistics teams to monitor every step involved in shipping antibodies—tracking temperatures, verifying documentation, and ensuring compliance with industry regulations. By leveraging real-time data analytics within this framework, organizations can proactively address potential issues before they escalate into costly problems.
Find more about R&D project management software.
Neotrident’s Unique Features in Carrier Compliance Audits
Let’s break down what makes Neotrident stand out when it comes to carrier compliance audits:
- Comprehensive Tracking: Neotrident offers end-to-end visibility for shipments through advanced tracking technologies.
- User-Friendly Interface: The platform provides an intuitive dashboard where users can easily access audit reports and compliance metrics.
- Error Reduction: Automated alerts notify stakeholders about any deviations from established protocols during transit.
- Sustainability Focused: Neotrident emphasizes eco-friendly practices by optimizing routes based on carbon footprint analysis.
- Crisis Management Tools: In case of emergencies or unexpected delays, built-in contingency plans help mitigate risks effectively!
A Conclusive Overview
The antibody registration system plays a pivotal role in maintaining high standards during carrier compliance audits. By integrating cutting-edge technology like R&D project management software alongside platforms such as Neotrident, we ensure not only adherence to regulations but also enhance overall efficiency within our supply chains. As we continue pushing boundaries in biopharmaceutical logistics, I am genuinely excited about how these innovations will shape the future!